[Introduction]Inotuzumab ozogamicin (IO) is highly effective in the treatment of relapsed/refractory CD22-positive B-acute lymphoblastic leukemia (B-ALL). IO exerts the antileukemic effect via DNA single- and double-strand breaks induced by calicheamicin. Although IO has been reported to show more than 80% response rate, the duration of remission is only 4.6 months, suggesting the early acquisition of drug resistance. Thus, it is crucial to elucidate the mechanism of resistance to IO and overcome it. Polyadenosine diphosphate-ribose polymerase (PARP) inhibitors, such as olaparib and talazoparib, show the antitumor effect by inhibiting DNA repair. This study was conducted to enhance the antitumor effect of IO by inhibiting IO-induced DNA damage repair using PARP inhibitors. The study attempted to overcome the resistance of B-ALL cell lines developed to be IO-resistant.
[Methods]Ph-negative, CD22-positive B-ALL Reh cell line was used to determine the cytotoxicity of IO and PARP inhibitors. Inotuzumab ozogamicin was kindly provided by Pfizer. Six IO-resistant cell lines were newly established by long-term exposure of Reh cells to IO, followed by the limiting dilution. FACS analysis evaluated CD22 expression. WTS assay and Annexin-V positivity were used to determine the antileukemic effects. The Comet assay assessed the DNA repair function. The DNA microarray compared gene expression between Reh and IO-resistant Reh clones.
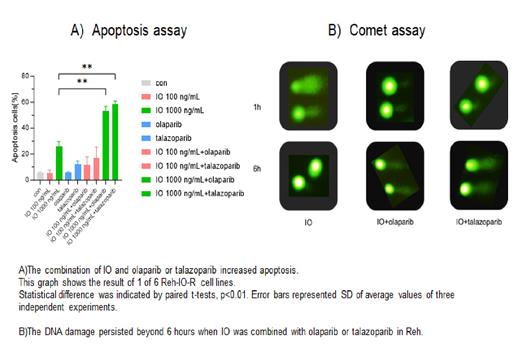
[Results] The growth inhibition effect of IO was significantly augmented by the addition of the minimally toxic concentration of olaparib or talazoparib in Reh cells. The combination index values were 0.19 and 0.42, respectively, demonstrating a strong synergism. Combining IO with olaparib or talazoparib increased IO-induced apoptosis compared to single-agent IO. The apoptosis rates induced by 20 ng/mL IO with minimally toxic concentrations of olaparib or talazoparib were 51.7% and 66.1%, respectively, showing significantly higher apoptosis rates than IO alone (23.5%). Assessment of DNA strand breaks using the Comet assay showed that the recovery of IO-induced DNA damage was inhibited in the presence of PARP inhibitors, and DNA strand breaks persisted. Next, six IO-resistant Reh (Reh-IO-R) cell lines were established as described above. The expressions of CD22 were more than 60%. All 6 cell lines had more than 60-fold resistance to IO with the 50%-inhibitory concentration (IC 50) values 474.3~782.3 ng/mL, compared with that of Reh cells (2.1 ng/mL). The IC 50 values of olaparib or talazoparib were similar to Reh and Reh-IO-R cell lines. When IO was combined with olaparib or talazoparib, the growth of Reh-IO-R cells was inhibited, with median IC 50 values were 359.4[290.3-406.2] and 179.1[59.3-240.5] ng/mL, respectively. This combination also remarkably increased apoptosis induction in Reh-IO-R cells. The apoptosis rate of Reh cells at 100 ng/mL IO alone was 77%, whereas the apoptosis rate of 6 Reh-IO-R cell lines averaged 5.9% [5.7-6.6%]. The median percentage of apoptosis in Reh-IO-R cell lines was 12.0% by IO + olaparib and 17.1% by IO + talazoparib. Furthermore, synergism was obtained in Reh-IO-R cell lines with the combination of 1,000 ng/mL IO with olaparib or talazoparib. The median percentage of apoptotic cell death was 24.2% by IO 1,000 ng/mL alone and 45.3% by the IO+olaparib, and 56.8% by the IO+talazoparib. These results suggest that the potentiating effect of PARP inhibitors would be attributed to more DNA cleavage occurring. The expression of genes ( ATM, ATR, DNA-PK, CHK1/2, PARP, RAD, ERCC1, BRCA1/2 Ku70/80) related to DNA repair was similar between Reh and Reh-IO-R cells. Of note, ABCB1 (p-gp) was overexpressed in Reh-IO-R cells. These results suggest that the expression of p-gp was one of the mechanisms of the resistance to IO, and the combination of IO and PARP inhibitors was effective to a certain extent in the Reh-IO-R cells expressing p-gp. This would be because the PARP inhibitors were still effective in inhibiting the DNA repair in Reh-IO-R cells where the DNA repair function was not augmented.
[Conclusion]The combination of IO with PARP inhibitors synergized the antileukemic effect of IO by inhibiting DNA strand break repair in CD22-positive ALL cell lines and IO-resistant ALL cells, suggesting that these results may contribute to overcoming the IO resistance mechanisms.
Disclosures
Hosono:Abbvie: Honoraria. Yamauchi:Astellas: Honoraria, Research Funding; Nihon Kayaku: Honoraria, Research Funding; Janssen Pharma: Honoraria, Research Funding; Nihon Shinyaku: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Sumitomo Pharma: Honoraria, Research Funding; Chugai: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal